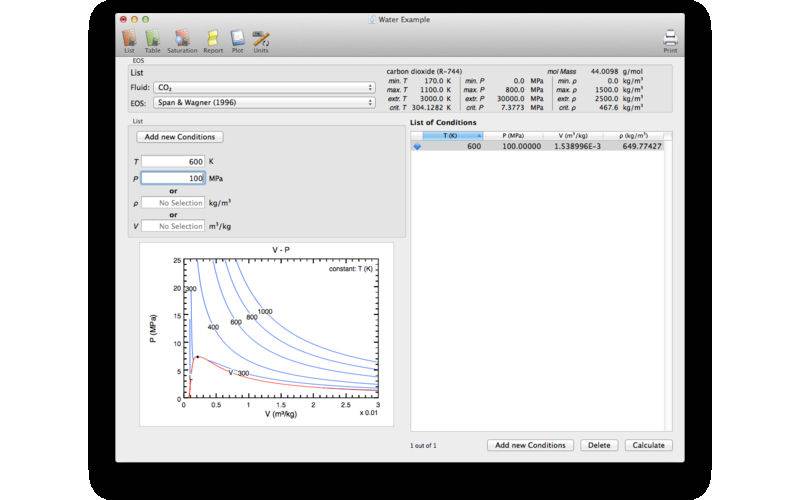
PureFluids calculates the Helmholtz free energy for various pure fluids using equations of state (EOS) collected from the scientific literature. Derivatives of the Helmholtz free energy with respect to density and temperature allows the calculation of the following thermodynamic properties:
inner energy, enthalpy, Helmholtz free energy, Gibbs free energy, entropy, heat capacity at constant V and P, ideal heat capacity at constant P, speed of sound, isobaric expansion coefficient, relative pressure coefficient, isothermal compressibility, Joule Thompson coefficient, isentropic temperature-pressure coefficient, isothermal throttling coefficient, isothermal stress coefficient, second virial and third coefficient, fugacity and fugacity coefficient, compressibility factor.
The program allows the calculation of the thermodynamic properties at arbitrary temperature and density or pressure conditions (List), tables with even spaced increments of temperature and density/volume (Table), or properties at the vapor saturation curve (Saturation). All these results are listed in Report tab which can be printed. The Plot tab allows the graphical representation of the thermodynamic properties for various conditions and includes the calculation of isopleths. These plots can be also printed. The Unit tab allows the user to choose the appropriate units for the individual thermodynamic properties.
By default the thermodynamic values for the inner energy, the enthalpy, the entropy, the Helmholtz free energy, and the Gibbs free energy are calculated as reported by the respective EOS. If required, the values for these properties can be corrected for an ideal gas with H = 0 and S = 0 at respective reference conditions (typically 298.15 K and 0.1 MPa). The reference conditions, however, can be chosen by the user.
Lists, normal and saturation tables, and plots can be also exported. The Help menu provides all the references for the respective EOS.
Currently the program includes 40 pure fluid species with 56 EOS. Some species are covered by several EOS. The following fluids are implemented:
simple fluids:
argon: Ar (R-740)
methane: CH₄ (R-50)
carbon monoxide: CO
carbonyl sulfide: COS
carbon dioxide: CO₂ (R-744)
hydrogen: H₂ (R-702)
parahydrogen: H₂-para
orthohydrogen: H₂-ortho
helium: He (R-704)
water: H₂O (R-718)
hydrogen sulfide: H₂S
krypton: Kr
nitrogen: N₂ (R-728)
ammonia: NH₃ (R-717)
nitrous oxide: N₂O (R-744A)
neon: Ne (R-720)
oxygen: O₂ (R-732)
sulfure dioxide: SO₂ (R-764)
xenon: Xe
more complex fluids:
fluoromethane : CH₃F (R-41)
hexafluoroethane: C₂F₆ (R-116)
ethane: C₂H₆ (R-170)
1,1-dichloro-1-fluoroethane : C₂H₃Cl₂F (R-141b)
1-chloro-1,1-difluoroethane: C₂H₃ClF₂ (R-142b)
propane: C₃H₈ (R-290)
1,1,1,3,3-pentafluoropropane: C₃H₃F₅ (R-245fa)
octafluoropropane: C₃F₈ (R-218)
2-propanone: C₃H₆O (acetone)
butane: C₄H₁₀ (R-600)
2-methylpropane: C₄H₁₀ (isobutane, R-600a))
pentane: C₅H₁₂ (R-601)
2-methylbutane: C₅H₁₂ (isopentane)
2,2-dimethylpropane: C₅H₁₂ (neopentane)
hexane: C₆H₁₄
2-methylpentane: C₆H₁₄ (isohexane)
methylbenzene: C₆H₅CH₃ (toluene)
heptane: C₇H₁₆
octane: C₈H₁₈
nonane: C₉H₂₀
decane: C₁₀H₂₂